
University of Colorado researchers are currently developing a saliva-based test that knows if you’re infected with a disease before symptoms even show. (Photo courtesy of University of Colorado)
As the novel coronavirus pandemic unites experts in finding ways to help flatten the curve, a team of University of Colorado Boulder researchers may have a game-changing solution: a test that knows if you’re sick before you do.
Darwin Biosciences, a new Boulder-based startup company, is bringing together scientists, a healthcare industry expert and a business guru to introduce early disease detection tests and, eventually, release the SickStick to the market.
“The problem with this virus (is) when you get infected, oftentimes you can be asymptomatic, meaning you never develop symptoms but you can be infectious and contagious,” said Nicholas Meyerson, a scientist in CU Boulder’s Department of Molecular, Cellular and Developmental Biology and CEO of Darwin Biosciences. “Even for those people that develop symptoms, they are in an incubation stage where they can be infectious and contagious without knowing it for upwards of a week.”
The SickStick aims to inform people of infection early in the process, working much like an at-home pregnancy test. Researchers say the test can detect a range of infections including SARS-CoV-2, the disease caused by the new coronavirus.
According to Meyerson, cells in the body have ways of detecting infection and send off “smoke signals” about a day after an infection that activates the body’s earliest immune response. The SickStick works when a user spits into a receptacle and inserts a strip that absorbs the saliva and displays a series of lines to indicate if that user is ill or not.
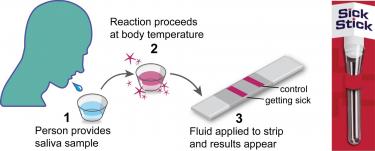
The SickStick aims to be a simple, three-step process that gets you quick results. (Image courtesy of University of Colorado)
“We can use saliva in our test as a non-invasive biospecimen,” Meyerson said. “You’ll see one red line if you’re healthy, two red lines if you’re getting sick, and since the pandemic broke out we’ve actually been able to incorporate a third line that specifically says ‘not only are you getting sick but you’re getting sick with (SARS-CoV-2).’”
This tool would be key to identifying who poses a risk to the public and who can return to work thanks to early detection—something current tests are unable to do.
“(Current) tests are great for diagnosing you if you’re infected and if you have symptoms and right now they are mostly being used on people that already present themselves to the hospital,” Meyerson said.
The project has already garnered positive attention, with the team taking top honors at CU’s 2020 New Venture Challenge pitch contest earlier this month. They won $30,000 in prize money and an additional $25,000 investment.
“It touches on so many different aspects of our society in terms of protecting vulnerable populations from potential infection (and) helping doctors make a more informed decision,” said Darwin Biosciences COO Rick Whitcomb, who is helping bridge the science and business worlds for the project.
In 2008, Whitcomb became president and CEO of Boulder-based company BiOptix, a molecular kinetics company, which he sold in 2016. He also mentored in CU’s Entrepreneur In Residence program, then serving as COO of a liver diagnostics company, utilizing technology that came out of CU’s Anschutz Medical Campus.
“One of (the) things that is challenging, I think, for scientific founders when you’re bringing a product to market in a regulated market … there’s quite a lot of regulatory infrastructure that you have to conform to,” Whitcomb said.
The research the project is based on has already received support from regulatory bodies, with the United States Department of Defense granting project co-founder Sara Sawyer a $3 million grant in 2018.
Sawyer is currently a virologist at the CU Boulder’s BioFrontiers Institute where she runs a lab studying the intersection of bioinformatics, genomics and evolutionary theory in order to understand human and animal viruses. Long before COVID-19 gripped the world, Sawyer and Meyerson worked together to better understand the body’s early immune response to infection and began developing the technology to be used as a quick, early diagnostic tool on military bases.
It may still be nine to 12 months before the at-home SickStick test is available on the shelves. Meyerson said he and his team are still in the development stage and will need to get FDA approval through clinical trials before anything goes to market. But, the team is working on bringing their early biomarker technology to clinical SARS-CoV-2 tests on an earlier timeline.
“What we really hope is that SickStick will be ready in the fall or winter when this pandemic is likely to reemerge,” Meyerson said. The lab is currently working on prototypes and hopes the mass production of the SickStick will be about nine months to a year from now.
Darwin Biosciences looks still to the future of their technology, hoping to develop more specific indications of viral versus bacterial infections to curb the over-prescription of antibiotics.
“The COVID piece is really important right now, and we’re committed to figuring out how we can help, but it’s going to go well beyond that, and I think that’s where the opportunities remain for us as a company,” Whitcomb said.
Contact CU Independent Staff Writer Mairead Brogan at mairead.brogan@colorado.edu.
